2016-02-23
Equine Cushing’s Syndrome
Cushings Syndrome has many “names” –pituitary pars intermedia dysfunction (PPID), hyperadrenocorticism, ECD (equine Cushing’s disease), and, most commonly, Cushing’s syndrome. Classically, this ailment occurs in geriatric horses however Cushings can be diagnosed in horses less than ten years old. Why is this medical condition becoming prevalent? The answer is straightforward, horses are living longer lives!
How does Cushings Syndrome develop and what is the function of cortisol?
The process begins when melantrope cells in the pars intermedia area of the pituitary become overgrown and overactive. They produce excess quantities of a peptide called pro-opiolipomelanocortin (POLMC, mercifully, for short). POLMC influences the adrenal glands, which reside on top of the kidneys, to produce an important hormone called cortisol. Cortisol has many functions;
- maintains blood pressure and cardiac function;
- reduces the immune system’s inflammatory response;
- regulates nerve tissue function, muscle tone, and connective tissue repair;
- balances the effects of insulin in breaking down sugar for energy;
- regulates the metabolism of carbohydrates, proteins, and fats;
- helps the body respond to stress.
Under normal circumstances, cortisol production is balanced by the hormone CRH (corticotropin releasing hormone) from the hypothalamus, which stimulates ACTH (adrenocorticotropic hormone) from the pituitary. ACTH in turn normally stimulates the release of cortisol. When there is enough cortisol in the bloodstream, ACTH and CRH secretion “backs off.” Pituitary adenomas result in excessive secretion of POLMC. Therefore, cortisol levels system can rise dramatically, producing the symptoms of Cushing’s.
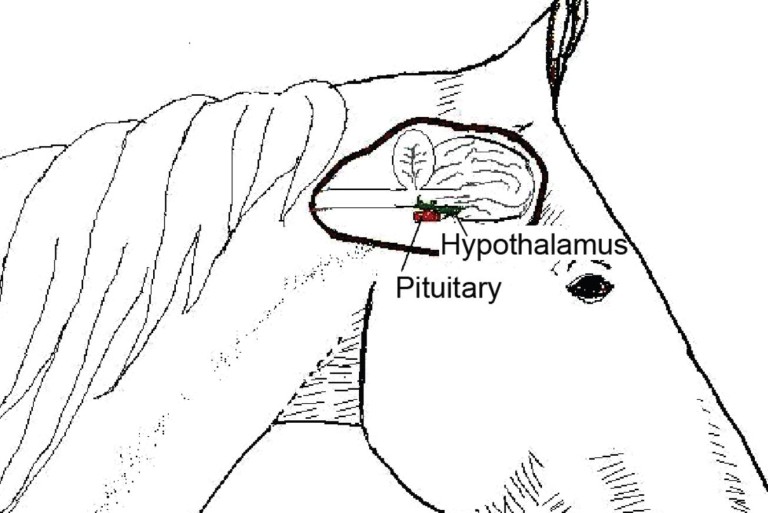
Anatomic diagram showing the location of the equine hypothalamus and Pituitary retrieved from http://d1uhp0uy75me04.cloudfront.net/mmah/d8/63a1783bd2449f831bc5f76848d087/fileVT0610_hembroff_CE.pdf
Clinical Signs

1. Increased water consumption and urination,
2. Development of a long and curly hair coat, Subtle hair coat changes, such as development of patchy areas of long hairs and a tendency for the winter hair coat to come in earlier and shed out later than normal, may precede more profound changes by many years.
3. Excessive sweating
4. Milk production in mares
5. Chronic infections such as sinusitis
6. Dental disease
7. Hoof abscesses
8. Laminitis and founder
9. Mares with Cushing’s disease often have reproductive problems such as complete failure to cycle, irregular estrous cycles, estrus suppression, decreased follicle development, ovulation failure, and reduced fertility
10. Presumably affected stallions would exhibit decreased sperm production and decreased conception rates in mares bred.
Diagnosis
Diagnosis of Cushing’s disease is usually based on the combination of clinical signs and blood tests. Affected horses may have elevated levels of glucose, insulin, cortisol and ACTH in their blood. A CBC (complete blood count) blood test is utilized to identify hyperglycemia–affected horses may have blood sugar levels over 120 mg per dl, and sometimes even greater than 300 mg per dl. Urinalysis also can be performed to detect glucosuria and ketonuria (abnormally high levels of glucose and ketones in the urine). The veterinarians at Arizona Equine frequently repeat these blood tests and urinalysis to establish the consistency of high blood sugar. This may prompt our veterinarians to perform more specific, hormone-related tests to confirm the diagnosis of Cushing’s syndrome.
The next test for Cushings is the dexamethasone suppression test and is the ‘gold standard’ for diagnosis of Cushing’s disease. Administration of dexamethasone (synthetic cortisol) to normal horses causes suppression of blood cortisol, whereas horses with Cushing’s disease have little to no change in cortisol levels in response to dexamethasone. The dexamethasone suppression test (DST) is one of the most commonly utilized tests. The “Dex-suppression test” is as follows; dexamethasone is injected intramuscularly into the horse after a baseline blood sample has been drawn. Nineteen to 24 hours later, another blood sample is drawn and tested for cortisol levels. A drop in cortisol level is the normal response (indicating that ACTH-producing cells in the pituitary have responded to normal feedback mechanisms). In a Cushing’s horse, however, the ACTH production will decrease in response to the dexamethasone injection, but the diseased melantrope cells will continue to produce peptides, creating a response of elevated cortisol.
Additional diagnostic tests include evaluation of cortisol levels in blood samples collected 8 to 10 hours apart or measurement of cortisol levels before and after administration of dexamethasone or other hormones. Evaluation of cortisol rhythm is a can be utilized as screening test for the presence of Cushing’s disease and is often performed as an alternative to the dexamethasone suppression test in horses with a history of laminitis. However, the cortisol rhythm test may yield false positive or false negative results, and one needs to be cautious basing a diagnosis on this test alone.
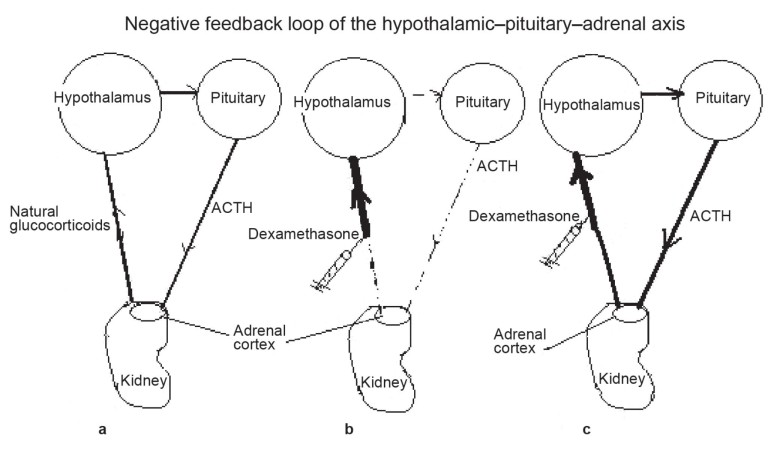
The hypothalamic–pituitary–adrenal axis creates a negative feedback loop that regulates homeostasis within the body. (a) In a healthy horse, the hypothalamus releases hormones that stimulate the pituitary to produce and release ACTH, which stimulates the production of glucocorticoids (cortisol) in the adrenal cortex. Exposure to an increased level of cortisol signals the hypothalamus to reduce its stimulation of the pituitary, reducing the release of ACTH and, in turn, lowering cortisol productionand maintaining homeostasis. (b) When dexamethasone (a synthetic form of cortisol) is given to a horse with a normally functioning pituitary, ACTH excretion is notably suppressed, as it would be if the horse were exposed to excessive endogenous cortisol, rapidly lowering the plasma cortisol level. (c) When dexamethasone is administered to a horse with PPID, the hyperplastic pars intermedia, which is poorly sensitive to the suppressive effect associated with hypothalamic exposure to cortisol, may continue to secrete a high level of ACTH. This stimulates the adrenal glands to continue releasing a high level of cortisol; therefore, the serum cortisol level is not suppressed.
Retrieved from http://d1uhp0uy75me04.cloudfront.net/mmah/d8/63a1783bd2449f831bc5f76848d087/fileVT0610_hembroff_CE.pdf
Treatment
Medical management of affected horses is usually a long-term or life-long commitment. Horses with Cushing’s disease require excellent management practices, including routine foot care, deworming, vaccinations, dentistry, and nutrition. Medical treatment regimens usually include administration of the dopamine agonist pergolide mesylate or the antiserotonin compound cyproheptadine. Clinical improvement, if any, is noted within 6 to 8 weeks after the onset of treatment. When an effective dose is established, the horse is maintained on that dose for life.
There are three drugs that have been used with success to reverse some or all of the symptoms of Cushing’s. All three operate on similar principles–they are either dopamine agonists, or serotonin antagonists. Dopamine and serotonin are two naturally occurring neurotransmitters in the brain, which help regulate the secretion of peptides like POLMC. One of dopamine’s functions is to inhibit melantrope cells in the pituitary; when dopamine levels are low, the melantrope cells become overactive. Serotonin performs the opposing function, stimulating the melantrope cells. Drugs that mimic the action of these neurotransmitters can, in essence, achieve the same aim from two different angles.
Bromocriptine mesylate (trade name Parlodel), a dopamine agonist, is the “original” drug used to treat Cushing’s syndrome. It mimics dopamine to inhibit overproduction of activating peptides, and it has been shown to mildly decrease plasma ACTH and cortisol levels. There is a problem with the drug, however, which limits its use–in an oral form, its absorption is poor, and the IM injectable form, which has to be administered twice a day, is impractical for long-term use.
A more successful choice is the serotonin blocker cyproheptadine. Available in an easily absorbed tablet form, cyproheptadine has been used to treat Cushingoid horses for a number of years
A third medication is pergolide mesylate (Prescend), a dopamine agonist that recently has become a very viable treatment alternative.
Conclusion
Horses whose symptoms are fairly mild definitely may respond best to the medication, and might have their useful lives extended by a number of years; but a horse which is already suffering from chronic founder and recurrent infections might derive limited benefit. It is worth remembering that none of these drug therapies addresses the root of the problem, the pituitary tumor itself. They merely treat the symptoms, and the tumor will continue to grow until at last it compromises the horse’s quality of life enough that euthanasia is the kindest answer. Caring for a horse with Cushing’s syndrome requires meticulous health management and preventative care. Key points are diet, vaccinations, deworming, hoof care and dentistry. Frequently these horses require body-clipping in the summer months to remove excessive coat. Treasured equine friends with Cushings can live a happy life if when their condition is well managed.
Contact Arizona Equine today if you have any further questions or are seeking treatment for your horse.
References
1. Schott II HC. Pituitary pars intermedia dysfunction: challenges
of diagnosis and treatment. Proc AAEP 2006.
2. McFarlane D. Role of the equine hypothalamic-pituitary pars
intermedia axis in health and disease. Proc AAEP 2006.
3. Lloyd DH, Littlewood JD, Craig JM, Thomsett LR. Practical
Equine Dermatology. Hoboken: Blackwell Publishing; 2008:106.
4. Donaldson MT, Jorgensen AJ, Beech J. Evaluation of suspected
pituitary pars intermedia dysfunction in horses with laminitis.
JAVMA 2004;224(7):1123-1127.
5. McFarlane D. Clinical forum: diagnosing pituitary pars intermedia
dysfunction. Compend Equine 2007;2(4):208-213.
6. McFarlane D. Diagnosing equine pituitary pars intermedia dysfunction
in ambulatory practice. Proc AAEP 2008.
7. Beech J, Boston RC, McFarlane D, Lindborg S. Evaluation of
plasma ACTH, α-melanocyte–stimulating hormone, and insulin
concentrations during various photoperiods in clinically normal
horses and ponies and those with pituitary pars intermedia dysfunction.
JAVMA 2009;235:715-722.
8. Messer IV NT, Johnson PJ, Ganjam VK. Diagnosis of pituitary
pars intermedia dysfunction: a review of 1999 versus 2008. Proc
AAEP 2008.

Sorry, the comment form is closed at this time.